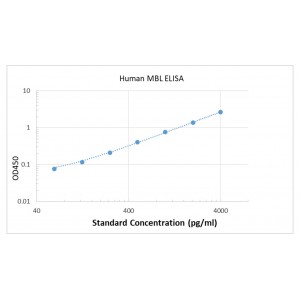
| Background |
Mannose-binding lectin (MBL), also called mannan-binding lectin or mannan-binding protein (MBP), is a lectin that is instrumental in innate immunity as an opsonin and via the lectin pathway. MBL has an oligomeric structure (400-700 kDa), built of subunits that contain three presumably identical peptide chains of about 30 kDa each. Although MBL can form several oligomeric forms, there are indications that dimers and trimers are biologically inactive as an opsonin and at least a tetramer form is needed for activation of complement. MBL belongs to the class of collectins in the C-type lectin superfamily, whose function appears to be pattern recognition in the first line of defense in the pre-immune host. MBL recognizes carbohydrate patterns, found on the surface of a large number of pathogenic micro-organisms, including bacteria, viruses, protozoa and fungi. Binding of MBL to a micro-organism results in activation of the lectin pathway of the complement system.
The complement system can be activated through three pathways: the classical pathway, the alternative pathway, and the lectin pathway. One way that the most-recently discovered lectin pathway is activated through mannose-binding lectin protein. MBL binds to carbohydrates (to be specific, D-mannose and L-fucose residues) found on the surfaces of many pathogens. It is produced in the liver as a response to infection, and is part of many other factors termed acute phase proteins. Expression and function in other organs were also suggested. The three structural polymorphisms of exon 1 have been reported to cause susceptibility to various common infections, including meningococcal disease. However, evidence has been presented that suggests no harmful effect of these variants with regard to mengingococcal disease.
|