More info
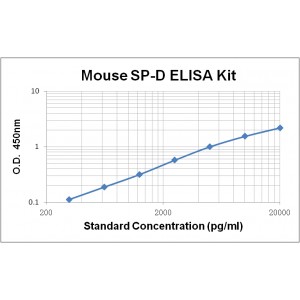
Assay Range | 312 -20,000 pg/mL |
Sensitivity | 10.0 pg/mL |
Size | 96T |
Storage | Store at 2 - 8ºC. Keep reconstituted standard and detection Ab at -20 ºC |
Assay Principle | Sandwich ELISA |
Sample Volume | 100 µL final volume, dilution factor varies on samples |
Detection Method | Chromogenic |
Kit Components
1. Recombinant Mouse SP-D standard: 2 vials
2. One 96-well plate coated with Mouse SP-D Ab
3. Sample diluent buffer: 12 mL - 1
4. Detection antibody: 130 µL, dilution 1:100
5. Streptavidin-HRP: 130 µL, dilution 1:100
6. Antibody diluent buffer: 12 mL x1
7. Streptavidin-HRP diluent buffer: 12 mL x1
8. TMB developing agent: 10 mL x1
9. Stop solution: 10 mL x1
10. Washing solution (20x): 25 mL x1
Background
Surfactant Protein-D (SP-D), also known as SFTPD, collectin-7, Lung surfactant protein D or PSP-D, is a member of the collectin family of innate immune modulators. The full-length SP-D is a 375 amino acid (aa) precursor protein composed of a 20 aa signal peptide and a 355 aa mature protein. Structurally, the SP-D protein is characteristic of a collagen-like region and a C-terminal carbohydrate recognition domain (CRD). SP-D is constitutively secreted by alveolar lining cells and epithelium associated with tubular structures, and is inducible in cardiac smooth muscle and endothelial cells. SP-D is detected in serum and plasma as well as broncho-alveolar lavage (BAL) fluid and amniotic fluid. In mice, mature mouse SP-D shares 76% and 92% aa sequence identity with human and rat SP-D, respectively.
SP-D forms a glycosylated, disulfide-linked trimer with an α-helical coiled-coil structure and a "head" of three symmetrical CRDs. Each CRD recognizes the hydroxides of one monosaccharide. Trimerization allows for the discrimination of monosaccharide patterns which is specific to microbial pathogens. SP-D functions through both secreted and transmembrane proteins. It binds human neutrophil defensins, modulating influenza antiviral defense. It binds MD-2/LY96, a secreted protein that cooperates with Toll-like receptors (TLRs) in the response of macrophages to bacterial lipopolysaccharides (LPS) or cell wall components. It also binds macrophage CD14 and TLRs directly to block binding of LPS and down-regulating TNF-α secretion. Clinically, increased circulating SP-D has been associated with a series of pathological conditions in lung, such as idiopathic pulmonary fibrosis, interstitial pneumonia, pulmonary alveolar proteinosis, severe acute respiratory syndrome (SARS), and chronic obstructive pulmonary disease (COPD). In addition, decreased SP-D in BAL fluid may be detectable in cystic fibrosis, SARS patients, and chronic smokers.