Data sheet
| Size | 20 µg |
| Catalog Number | PR-C1001 |
| Supplied In | 100 µL of 0.2 mg/mL Carbamylated BSA in 1X PBS containing 0.05 mM EDTA, 0.02 % NaN3, 5% Glycerol |
| Applications | 1. Western Blot: use 0.1 to 1 µg of the protein as control. 2. ELISA: use 10 to 200 ng as positive control or blocker for anti-carp antibody ELISA assay |
| Protein concentration | 0.2 mg/mL |
| Storage/ Stability | -20ºC . 1 year from date of receipt under proper storage conditions; aliquot to avoid multiple freeze thaw cycles. |
More info
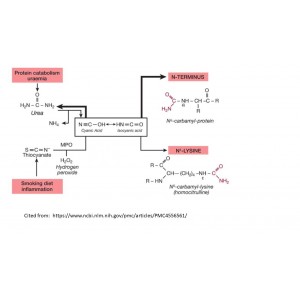
Protein carbamylation refers to the posttranslational modification of proteins or amino acids via adduction with isocyanic acid, such as on either the N-terminus of proteins or free amino acids (Nα-carbamylation) or the Nε-amino group of protein lysine residues forming carbamllysine (homocitrulline). Isocyanic acid is formed through either spontaneous decomposition of urea, or myeloperoxidase (MPO) catalyzed oxidation of thiocyanate at sites of inflammation, including atherosclerotic plaques.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4556561/
Related Products:
1. Human Anti-Carbamylated Protein Antibody, ( Anti-CarP) ELISA Kit, Cat#: BG-HUM09021
2. Rabbit anti-CarP antibody, Cat# AB-09021R
3. Carbamylated protein ELISA, Cat# BG-HUM09021P
Several proteins have been demonstrated to undergo carbamylation in different pathophysiological conditions, often altering their structure and rendering them dysfunctional. Long-lived proteins are particularly prone to PTMs such as carbamylation, which are considered the hallmark of molecular aging. Carbamylation of a-crystallins induces conformational changes responsible for lens opacities in cataract. In addition, carbamylation disturbs the triple helix structure of collagen type I, leading to a decreased ability to polymerize into normal fibrils and increased susceptibility to collagenases. Furthermore, enzymatic activity of insulin and erythropoietin are substantially diminished after carbamylation. Interestingly, carbamylation has also been shown to be potentially involved in the pathogenesis of rheumatoid arthritis, where in animal models carbamylated peptides were shown to serve as a potent neo-antigen for production of autoantibodies and an erosive arthritis phenotype. Importantly, recent studies also show protein carbamylation occurs at increased levels within atherosclerotic plaques1, and alternative studies suggest that protein carbamylation may play a role in Alzheimer disease development through the generation of abnormal tau protein deposits in the brain.