More info
|
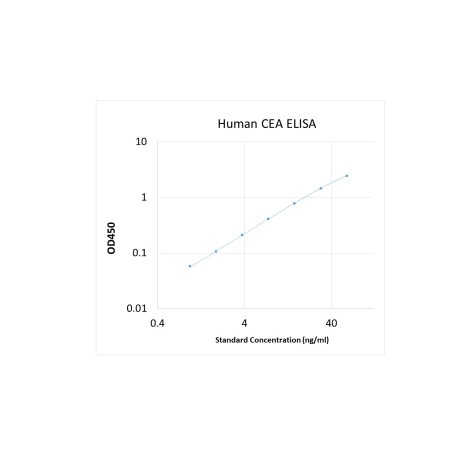
Assay Range |
0.75 -60 ng/mL |
|
Sensitivity |
0.2 ng/mL |
|
Size |
96T |
|
Storage |
Store at 2 - 8ºC. Keep reconstituted standard and detection Ab at -20 ºC |
|
Assay Principle |
Sandwich ELISA |
|
Sample Volume |
100 µL final volume, dilution factor varies on samples |
|
Detection Method |
Chromogenic |
Kit Components
•
Antibody-coated microtiter plate, 96 wells
•
Enzyme Conjugate Reagent
12 ml
•
TMB Substrate
12 ml
•
Stop Solution
12 ml
•
Reference standards, containing 0, 0.75, 1.5, 3, 6, 15, 30, 60 ng/ml of CEA, in
liquid form (ready to use)
•
Wash Buffer Concentrate (50X),
15 ml
Background
Carcinoembryonic antigen (CEA) is a protein group of highly related glycoproteins involved in cell adhesion. CEA is normally produced in gastrointestinal tissue during fetal development, but the production stops before birth. In adults, CEA is expressed only in cancer cells, and the serum levels are raised in some types of cancer. It is proposed that CEA may be used as a tumor marker. CEA are glycosyl phosphatidyl inositol (GPI) cell surface anchored glycoproteins whose specialized sialofucosylated glycoforms serve as functional colon carcinoma L-selectin and E-selectin ligands, which may be critical to the metastatic dissemination of colon carcinoma cells. It is reported that serum from individuals with colorectal carcinoma often has higher levels of CEA than healthy individuals. CEA levels may also be raised in gastric carcinoma, pancreatic carcinoma, lung carcinoma, breast carcinoma, and medullary thyroid carcinoma, as well as some non-neoplastic conditions like ulcerative colitis, pancreatitis, cirrhosis, COPD, Crohn's disease, hypothyroidism as well as in smokers. Most types of cancer do not result in a high CEA levels, however, the CEA blood test is not reliable for diagnosing cancer or as a screening test for early detection of cancer.