 View larger
View larger
Human Cholesteryl Ester Transfer Protein CETP ELISA Kit
BG-HUM10544
Human Cholesteryl Ester Transfer Protein (Cetp) ELISA KitFor research use only. Not for use in diagnostic procedures.
Data sheet
| Assay time | 2.5 hours |
| Validity | 6 months |
| Storage | see manual |
| Application | ELISA |
| Background | Cholesteryl ester transfer protein (CETP), also called plasma lipid transfer protein, is a plasma protein that facilitates the transport of cholesteryl esters and triglycerides between the lipoproteins. It collects triglycerides from very-low-density (VLDL) or low-density lipoproteins (LDL) and exchanges them for cholesteryl esters from high-density lipoproteins (HDL), and vice versa. Most of the time, however, CETP does a heteroexchange, trading a triglyceride for a cholesteryl ester or a cholesteryl ester for a triglyceride. Rare mutations leading to reduced function of CETP have been linked to accelerated atherosclerosis.In contrast, a polymorphism (I405V) of the CETP gene leading to lower serum levels has also been linked to exceptional longevity and to metabolic response to nutritional intervention. However, this mutation also increases the prevalence of coronary heart disease in patients with hypertriglyceridemia. The D442G mutation, which lowers CETP levels and increases HDL levels also increases coronary heart disease. As HDL can alleviate atherosclerosis and other cardiovascular diseases, and certain disease states such as the metabolic syndrome feature low HDL, pharmacological inhibition of CETP is being studied as a method of improving HDL levels. To be specific, in a 2004 study, the small molecular agent torcetrapib was shown to increase HDL levels, alone and with a statin, and lower LDL when co-administered with a statin. Studies into cardiovascular endpoints, however, were largely disappointing. While they confirmed the change in lipid levels, most reported an increase in blood pressure, no change in atherosclerosis, and, in a trial of a combination of torcetrapib and atorvastatin, an increase in cardiovascular events and mortality. |
More info
Human Cholesteryl Ester Transfer Protein (Cetp) ELISA Kit
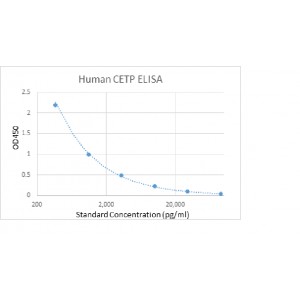
Assay range: 370 - 90,000 pg/ml
Sensitivity: 100 pg/ml
Reporducibility: Intra-Assay: CV<8%; Inter-Assay: CV<12%
Sample Reference value CETP (Shimada et al. Lipids in Health and Disease (2016) 15:57 DOI 10.1186/s12944-016-0223-6): 2.54 +/- 0.60 ug/ml
Reference:
1. Izem L, Morton RE. Cholesteryl ester transfer protein biosynthesis and cellular cholesterol homeostasis are tightly interconnected. J Biol Chem. 2001;276:26534–41.
2. Chapman MJ, Le Goff W, Guerin M, Kontush A. Cholesteryl ester transfer protein: at the heart of the action of lipid-modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer proteininhibitors. Eur Heart J. 2010;31:149–64.
3. Quinet E, Tall A, Ramakrishnan R, Rudel L. Plasma lipid transfer protein as a determinant of the atherogenicity of monkey plasma lipoproteins. J Clin Invest. 1991;87:1559–66.
4. de Grooth GJ, Smilde TJ, Van Wissen S, Klerkx AH, Zwinderman AH, Fruchart JC, et al. The relationship between cholesteryl ester transfer protein levels and risk factor profile in patients with familial hypercholesterolemia. Atherosclerosis. 2004;173:261–7.
5. Honzumi S, Shima A, Hiroshima A, Koieyama T, Ubukata N, Terasaka N. LXRalpha regulates human CETP expression in vitro and in transgenic mice. Atherosclerosis. 2010;212:139–45.
6. Luo Y, Tall AR. Sterol upregulation of human CETP expression in vitro and in transgenic mice by an LXR element. J Clin Invest. 2000;105:513–20.
7. Beltowski J. Liver X, receptors (LXR) as therapeutic targets in dyslipidemia. Cardiovasc Ther. 2008;26:297–316.
8. Jonker JT, Wang Y, de Haan W, Diamant M, Rijzewijk LJ, van der Meer RW, et al. Pioglitazone decreases plasma cholesteryl ester transfer protein mass, associated with a decrease in hepatic triglyceride content, in patients with type 2 diabetes. Diabetes Care. 2010;33:1625–8.

